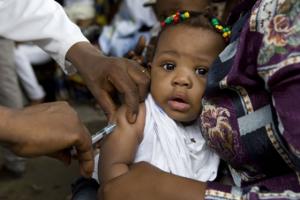
Meningitis A vaccine now recommended in routine immunization schedules
Brazzaville, 20 January 2015 – The World Health Organization (WHO) now recommends the conjugate meningitis A vaccine MenAfriVac® to be introduced in routine immunization schedules in sub-Saharan Africa. This recommendation ensures that infants are protected against meningitis and population-wide immunity is maintained.
The use of the MenAfriVac® vaccine to prevent meningitis A epidemics is one of the greatest vaccination success stories in public health history and highlights what partners can accomplish when unified by a compelling cause. In 2014, the MenAfriVac® campaigns reached more than 63 million people with remarkable success.
In all, over 217 million people between one and 29 years of age have benefited from the vaccine since 2010.
This unprecedented achievement is due to the overall availability, safety and effectiveness of the vaccine. MenAfriVac® is incredibly stable – being the first vaccine to be used with the controlled temperature chain (CTC) approach. This has allowed its transport and storage for as long as four days in ambient temperatures up to 40°C. This shattered preexisting cold chain limitations and paved the way to effectively deliver the vaccine to the hardest to reach people in need.
In October 2014, the WHO Strategic Advisory Group of Experts (SAGE) on immunization concluded that a one-dose schedule at nine months of age or older is recommended. At least six countries (Burkina Faso, Chad, Ghana, Mali, Niger, and Nigeria) are planning to introduce MenAfriVac® into their routine immunization programmes by late 2015.
“MenAfriVac® has already had a dramatic impact on breaking the cycle of meningitis A epidemics and now the prequalification of the vaccine in children less than one year of age ensures a highly effective, affordable, and sustainable solution to protect new cohorts of infants in countries affected by meningitis,” says Dr Luis Sambo, Regional Director for Africa.
Despite tremendous progress, countries cannot become complacent as bacterial meningitis remains a serious threat to more than 400 million people – especially infants and young children – in 26 countries along the ‘African meningitis belt’ that extends from Senegal to Ethiopia.
Meningitis is an infection of the thin lining that surrounds the brain and spinal cord called the meninges. Viral and bacterial infections are the most common cause but bacterial meningitis is much more serious due to its rapid onset and poses a significant risk of death.
Bacterial meningitis may also result in mental retardation, deafness, epilepsy, or necrosis leading to limb amputation. It is important to know which type of bacteria is causing the meningitis because antibiotics can prevent some types from spreading and infecting other people.
Significant progress in the fight against meningitis has been achieved through support from Gavi, the Vaccine Alliance, WHO, UNICEF, PATH, the Bill & Melinda Gates Foundation, the Michael and Susan Dell Foundation, the Shefa Fund hosted by the Swiss Philanthropy Foundation, the National Philanthropic Trust, the Giving Fund hosting generous individual donors funds, CDC, MSF, USAID and governments in sub-Saharan Africa. MenAfriVac® mass vaccination campaigns will continue in Ethiopia and in four new countries in 2015 (Democratic Republic of Congo, Guinea, Guinea Bissau and South Sudan). Burundi, Central African Republic, Eritrea, Kenya, Rwanda, Uganda and Tanzania will be the seven last African countries to conduct MenAfriVac® campaigns in 2016.
Millions of children remain unimmunized against vaccine-preventable diseases because either vaccines are not available, health systems fail to provide integrated services due to inadequate funding and human resources, or because families are uninformed or misinformed about the benefits of immunization.
The overall success of the MenAfriVac® vaccination campaign shows that it is possible to deliver vaccines more effectively and at a lower cost when refrigeration is not a major limitation. This has encouraged vaccine producers to find out-of-cold chain solutions that can be applied in other lifesaving vaccines such as human papillomavirus (HPV) and hepatitis B birth dose.
_________________________________________
For more information, please contact:
Technical contacts:
Dr Carol Tevi-Benissan
Tel: +472 413 9412
Email: tevibenissanc [at] who.int (tevibenissanc[at]who[dot]int)
Dr Mamoudou Harouna Djingarey
Tel: +472 413 7825
Email: djingareyh [at] who.int (djingareyh[at]who[dot]int)
Media contacts:
Dr Cory Couillard
Tel: + 472 413 9995
Email: couillardc [at] who.int (couillardc[at]who[dot]int)
Collins Boakye-Agyemang
Tel: + 472 413 9420
Email: boakyeagyemangc [at] who.int (boakyeagyemangc[at]who[dot]int)