Liberia embraces the Oral Polio Vaccine SWITCH
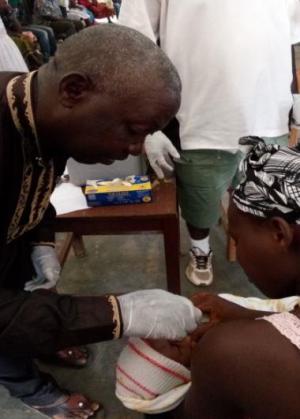
Monrovia 23rd April - The Ministry of Health Liberia embraced the move to switch from the use of trivalent oral polio vaccine (tOPV) to bivalent oral poliovirus vaccine bOPV in its routine immunization schedule. This initiative to withdraw type 2 component of tOPV is part of the global polio eradication endgame strategy for 2013-2018.
All of Liberia’s 15 counties successfully implemented the national SWITCH day on April, 22nd, 2016. This historical milestone comes three days before the regional launch of the African Vaccination Week in Ganta, Nimba County on 25th April.
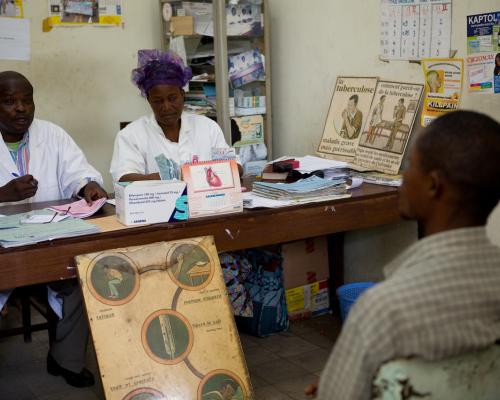
All counties have adequate supply of bOPV for routine immunization and have removed all tOPV stocks from the cold chain (i.e. walk-in cold rooms, freezers, refrigerators, cold boxes and vaccine carriers e.t.c). Health workers and supervisors across the country were adequately prepared for this activity through training. A coordination structure was also constituted at national, county and district levels with both government and partner representation to ensure that all systems are set for a successful transition.
To fulfill the SWITCH requirements County health teams developed micro-plans to help estimate the vaccine requirement for the initial 6 weeks plus buffer stock. The push method was used to ensure timely supply of bOPV to all health facilities with minimal interruption in service delivery.
According to Mrs Mary Momolu, National EPI Manager, “The SWITCH exercise has been a huge success. Reports from all the 15 counties indicate that the switch has been hitch free. I visited the some selected health facilities and observed that all tOPV vials have been removed from the cold chain, properly sealed and labeled in readiness for destruction”. Dr Badini Mehboob WHO Montserrado County Coordinator also confirmed that all the 118 HCFs (fixed sites) in the county had effectively removed the tOPV vials from their cold chain and health workers are now using the bOPV.
Independent monitoring of the switch has commenced today to conclude on April 28th covering the primary cold store at national level, all secondary cold stores at sub-national level and selected health facilities. Data will be submitted to the national switch validation committee who will analyze and compile a report to be submitted to the MoH by May 4th and subsequently to WHO by May 6th confirming successful switch exercise.
The move to withdraw tOPV was recommended by the World Health Assembly last year following confirmation that one of the three strains of wild poliovirus had been eradicated. The Global Commission for the Certification of Poliovirus Eradication declared in September 2015 that wild poliovirus type 2 had been eradicated globally. The risks associated with continued use of the type 2 component of tOPV outweighs the benefits and hence the rationale for its withdrawal.
________________________________________________
For more information please contact:
Technical: Dr Zakari Wambai: email wambaiz [at] who.int ( wambaiz[at]who[dot]int)
Communication: Luwaga Liliane: email: lluwagal [at] who.int ( lluwagal[at]who[dot]int)