AVAREF Overview
The African Vaccine Regulatory Forum (AVAREF) is a network of African national regulatory authorities and ethics committees that uses harmonization and reliance as pillars for capacity building.
AVAREF works to ensure collaboration between key stakeholders across the continent—including donors, health professionals, and regional economic blocs—by promoting joint reviews and the sharing of work and expertise.
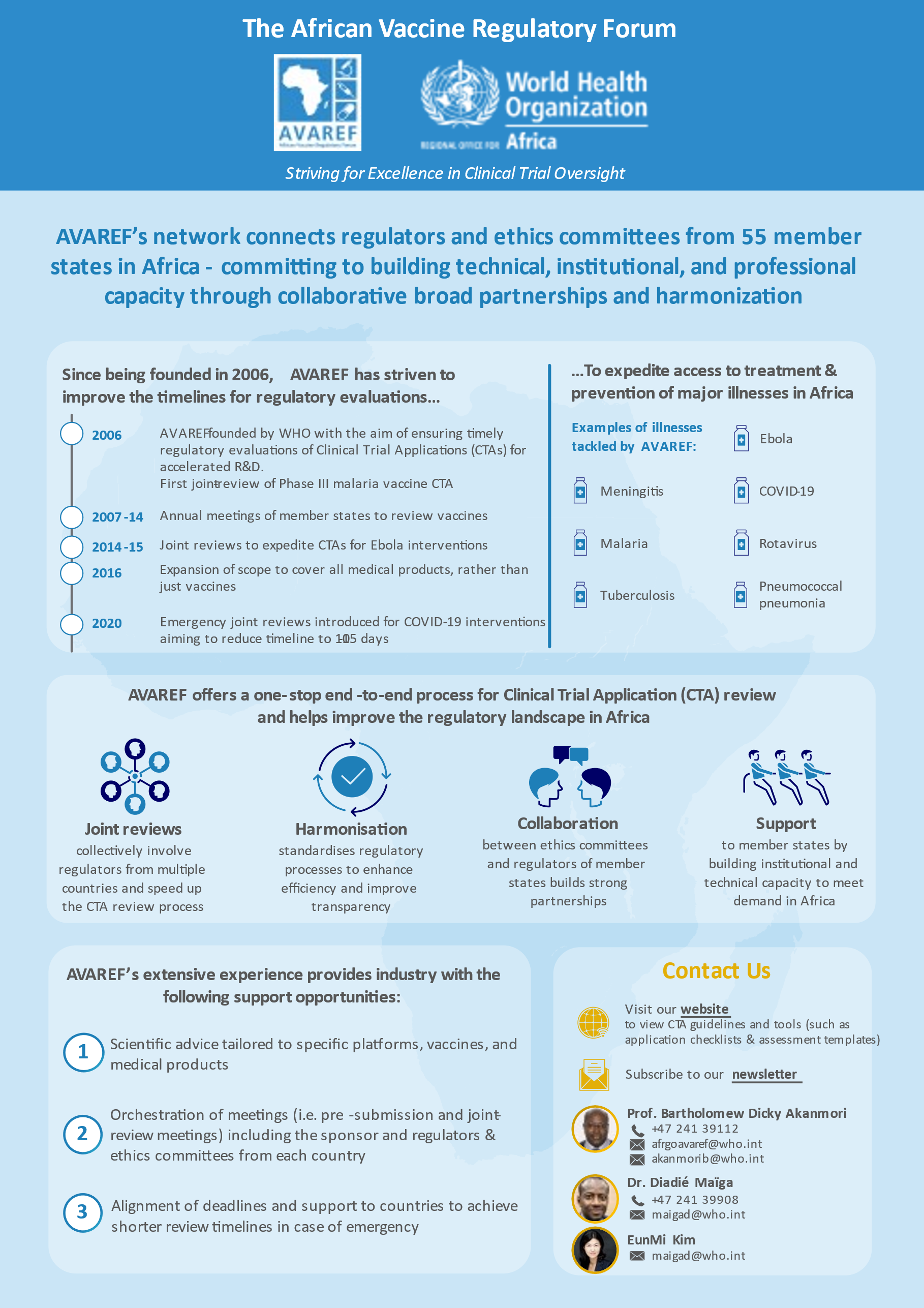
As a result of AVAREF’s efforts, vaccines against meningitis, malaria, rotavirus, pneumococcal pneumonia, and Ebola have been developed, and medicines against neglected diseases such as human African trypanosomiasis and leishmaniasis are currently being developed.
Misson
The African Vaccine Regulatory Forum (AVAREF) is a network of African national regulatory authorities and ethics committees that uses harmonization and reliance as pillars for capacity building.
AVAREF works to ensure collaboration between key stakeholders across the continent—including donors, health professionals, and regional economic blocs—by promoting joint reviews and the sharing of work and expertise.
As a result of AVAREF’s efforts, vaccines against meningitis, malaria, rotavirus, pneumococcal pneumonia, and Ebola have been developed, and medicines against neglected diseases such as human African trypanosomiasis and leishmaniasis are currently being developed.
History
AVAREF was established by the World Health Organization (WHO) in 2006 as a platform for building ethics and regulatory capacity for clinical trials in Africa and promoting the harmonization of ethics and regulatory processes on the continent. AVAREF emerged as the natural outgrowth of a 2006 joint review of a clinical trial application for a new group A meningococcal conjugate vaccine and the subsequent joint good clinical practice (GCP) inspection of the vaccine’s phase II trial. Since 2006, AVAREF has convened annual meetings to review clinical trial applications for vaccines against meningitis, malaria, tuberculosis, and other diseases.
Recognizing the pressing health needs on the continent, the AVAREF Assembly extended AVAREF’s governance and scope during its 2016 meeting in Addis Ababa, Ethiopia. While AVAREF’s initial focus was on vaccine clinical trials, its new mandate includes improving and harmonizing ethics and regulatory processes for clinical trials of vaccines as well as medical products and devices, in line with its 2018-2020 strategic plan. In addition, in 2019, AVAREF became one of the Continental Technical Committees of the African Medicines Regulatory Harmonisation (AMRH) Initiative. Once the new governance structure is implemented, AVAREF will play a significant role in supporting the African Medicine Agency to become better established.
Among many other activities, AVAREF has been working collaboratively with Member States and sponsors to expedite emergency joint reviews of clinical trial applications for products that target COVID-19.
AVAREF’s History at a Glance
| 2006 |
|
| 2007-2014 |
|
| 2014-2015 |
|
| 2016 |
|
| 2020 |
|
AVAREF Information Brochure
Download for One Pager Introduction of AVAREF (English)
Download for One Pager Introduction of AVAREF (Français)