AVAREF COVID-19 Response
During epidemics, pandemics, or other health emergencies, AVAREF can facilitate approvals of clinical trials and accelerate access to life-saving vaccines and medicines. Information on AVAREF’s timely COVID-19 Action plan and Guidance for Emergency Preparedness resources to help African countries during COVID-19 is included below.
AVAREF STRATEGY AND GUIDANCE FOR EMERGENCY PREPAREDNESS, INCLUDING DESCRIPTION OF EMERGENCY JOINT REVIEW PROCESS
This document guides ethics committees and national regulatory authorities as they undertake emergency reviews during a pandemic or epidemic. It provides sponsors and Africa’s regional economic communities with guidance on the critical elements of emergency preparedness, to ensure that ethics and regulatory decisions do not constitute barriers to access, but instead promote public health. The processes described are consistent with national emergency preparedness and response plans, as well as with local guidelines for public health emergencies.
The document contains a detailed description of the emergency joint review process for clinical trial applications, including a discussion of the application process and stakeholders’ roles and responsibilities. The timelines for emergency joint reviews are 10 working days for products already registered for other indications and 15 working days for novel products. These timelines encompass the entire review process, from AVAREF’s receipt of the clinical trial application to the joint review panel’s final decision. They apply to parallel submissions to ethics committees and national regulatory authorities as well, not counting clock stops. When the application goes back to the sponsor with questions to prepare responses to the regulatory authority, the period of time during which the evaluation of a medicine is officially stopped. The clock resumes when the applicant has sent its responses.
The document also provides guidance on importing investigational products and exchanging and transferring biological specimens. Finally, AVAREF recommends that emergency preparedness plans be tested at the level of individual countries, regional economic communities, and the continent.
THE AVAREF ACTION PLAN FOR COVID-19
The COVID-19 pandemic has now spread to all continents, and the World Health Organization (WHO) has designated it the most severe emergency the agency has ever declared. Researchers worldwide are now making massive efforts towards developing medicines and vaccines to fight the virus. The Ebola epidemic of 2014-2015 demonstrated that it is possible to accelerate research and development (R&D) during emergencies. It also showed that it is feasible to safely and effectively implement clinical trials for medicines, vaccines and other interventions in affected low-income countries. Leaning on this and other experiences, AVAREF has worked with its partners and the WHO Secretariat to develop a comprehensive COVID-19 Action Plan.
Why an AVAREF Action Plan for COVID-19?
AVAREF’s past work has demonstrated the importance of R&D preparedness and effective collaboration and partnership frameworks in combatting epidemics. In addition to facilitating the conduct of clinical trials of Ebola products, AVAREF approved and oversaw the development of a conjugate meningitis A vaccine that has drastically reduced meningitis A epidemics in Africa. Using the same basic approach, tailored to the unique circumstances of the COVID-19 pandemic, the AVAREF Action Plan aims to achieve the following:
- To complement the R&D component of the Emergency Response to COVID-19 in Africa
- To contribute to the Global Strategy and Preparedness Plan to accelerate COVID-19 product development
- To serve as a convening mechanism, to articulate technical guidance on clinical trials, and to promote knowledge-sharing on the African continent
Objectives
The AVAREF Action Plan will create a forum for R&D stakeholders to accelerate the development of products targeting COVID-19. It will fund partners; enhance the capacity of regulators and ethics committees in Africa; engage researchers, sponsors, and product developers; promote partnerships; and share information. The Action Plan identifies the following COVID-19 priority activities and deliverables:
- Host AVAREF virtual forums to engage product developers, scientists, and research centers in Africa
- Build the regulatory and ethics capacity of active AVAREF members and reach out to African countries that are not fully engaged in AVAREF, through weekly/monthly updates on COVID-19-related regulatory activities
- Convene the AVAREF Assembly (i.e., heads of national regulatory authorities and national ethics committees) to endorse guidance documents and recommendations that will accelerate the development of products for use against COVID-19
- Facilitate timely scientific advice meetings between COVID-19 product developers/sponsors and AVAREF members
- Facilitate expedited joint/assisted reviews of clinical trials for products targeting COVID-19
THE AFRICAN VACCINE REGULATORY FORUM (AVAREF) CONDUCTS FIRST MULTI-COUNTRY, MULTI-SPONSOR EMERGENCY JOINT REVIEW OF A COVID-19 CLINICAL TRIAL APPLICATION
Background
AVAREF aims to build capacity for clinical trials and to accelerate development vaccines and medicines, and has contributed to the development and licensure of several vaccines in the African region. To accelerate the development of diagnostics, vaccine and medicines addressing the COVID-19 pandemic, AVAREF Member States endorsed an emergency joint review process with 10 days for review of applications of registered products (e.g., chloroquine, hydroxychloroquine, remdesivir, ritonavir, etc.) and 15 days for novel products, (i.e., vaccines) (see Figure 1).
Figure 1: The AVAREF Emergency Joint Review Process
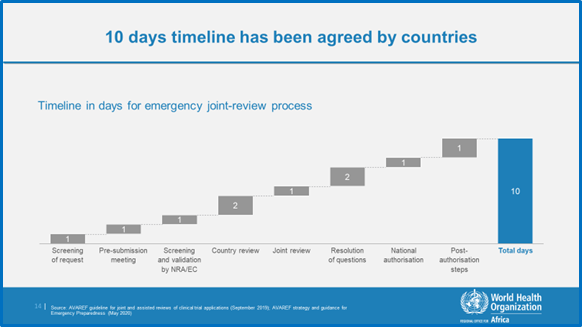
The joint review for an application to test antivirals against COVID-19 using this emergency process took place on 5-6 July 2020. The protocol was submitted to ethics committees and national regulatory authorities of Burkina Faso, Cameroon, the Democratic Republic of the Congo, Ethiopia, Ghana, Guinea, Ivory Coast, Kenya, Mali, Mozambique, Niger, Senegal, Sudan and Uganda. The clinical trial was sponsored by a consortium of sponsors from France, Spain and Germany, with investigator teams in all the countries, and coordinated by the Drugs for Neglected Diseases initiative (DNDi). This was the first ever virtual joint review meeting by AVAREF.
Electronic submission of clinical trial application packages in all countries was preceded by a pre-submission meeting, followed by the joint review meeting to reconcile queries raised and a final alignment meeting, all held virtually. The quality of the review was high, demonstrated by the 300 queries raised by the reviewers. Seventy percent of the countries delivered decisions in 25 days, the shortest timeline ever for a joint review by AVAREF. Five countries delivered decisions in 16 days, missing the target of 10 days by five days. At the end of the joint review, nearly 50% percent of the countries had met the deadline of 10 days, indicating their commitment and ability to review a clinical trial application and to issue a verdict within the 10-day timeline. Some countries failed to meet the timeline of 10 days, even though a very good review was conducted, mainly because decisions were delayed (see Table 1).
Figure 1: The AVAREF Emergency Joint Review Process
- 70% of authorities who participated in the full joint review were able to deliver decisions within 25 days or fewer
- 5 Countries were able to deliver decisions in 16 days or fewer
- 2 Countries were able to deliver decisions between 17-25 days
- 5 Countries took longer than 25 days to deliver decisions
- 2 of these countries did not fully participate in the joint review but relied on the review of those who fully participated.
Despite the successful implementation of the first emergency joint review, there were also challenges, including the inability of the countries to issue decisions and meet the timeline. Further exploration showed that the delays in ethics decisions and in some cases by National Regulatory Authorities (NRAs) were responsible for the unmet timelines. Another major cause of delay was additional queries raised by some countries outside the joint review process, in some cases ignoring adequate responses to queries which sponsors had provided during the joint review.
A detailed analysis is ongoing to improve the process because an emergency review process can contribute to accelerated development of vaccines, especially in epidemics and pandemics. The WHO AVAREF Secretariat is committed to supporting Ethical Committees (ECs) and NRAs of the countries of the region to implement such a process.
AVAREF information relevant to COVID-19
An online platform (Mednet Community) has been established to facilitate information-sharing on COVID-19 among AVAREF members. (Note to AVAREF Members: If you have not received an automatic email, please contact the Secretariat and let us know so that we can add you to the community: https://mednet-communities.net/avaref-covid-19/.)


