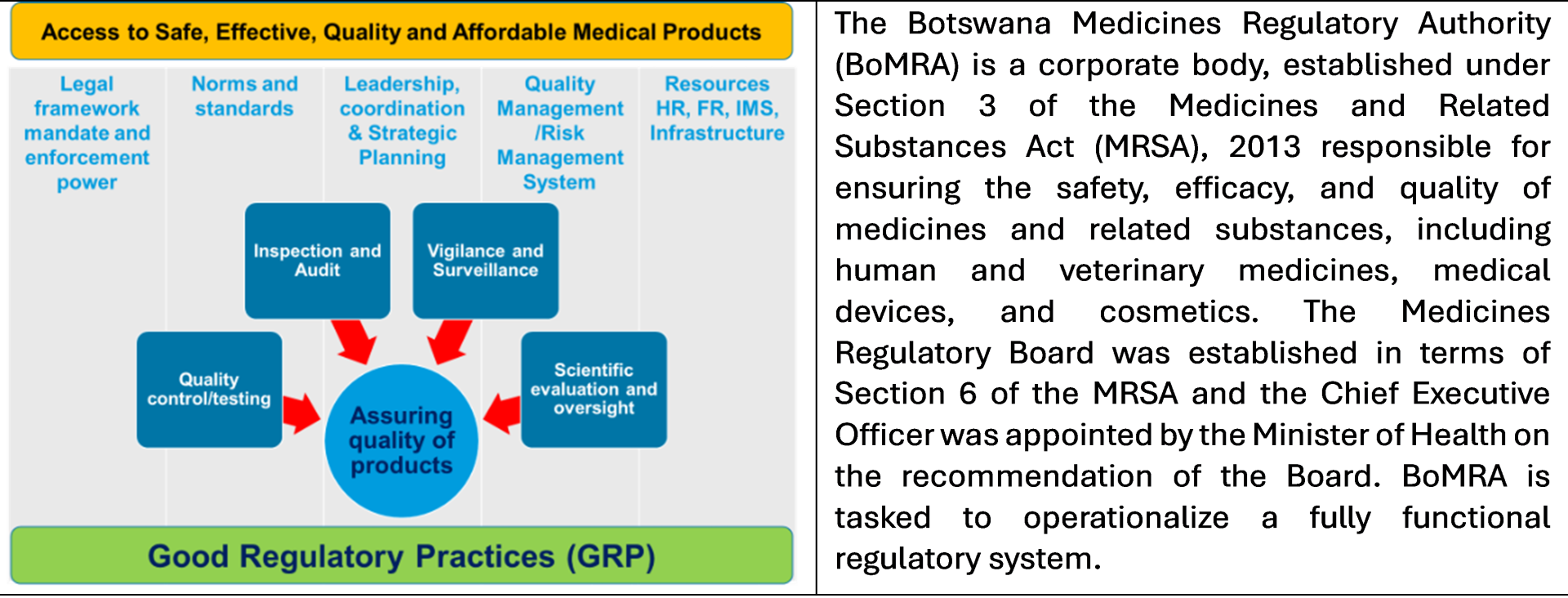
Botswana Medicines Regulatory Authority conducts five-day assisted self-benchmarking exercise
Gaborone—The Botswana Medicines Regulatory Authority (BoMRA) successfully concluded a five-day assisted self-benchmarking exercise from 11 to 15 August 2025, with the support of the World Health Organization (WHO). The assessment, conducted using the WHO Global Benchmarking Tool (GBT), reviewed all regulatory functions to guide BoMRA’s journey toward achieving WHO Maturity Level 3 (ML3), the global standard for an integrated, fully functional pharmaceutical regulatory system. Attaining WHO ML3 will be a critical milestone for the nation, guaranteeing even greater safety, quality, and effectiveness of medicines for everyone in Botswana. The WHO Team comprised of technical officers from the Regulatory Strengthening System programme at HQ/AFRO/WCO and independent consultants.
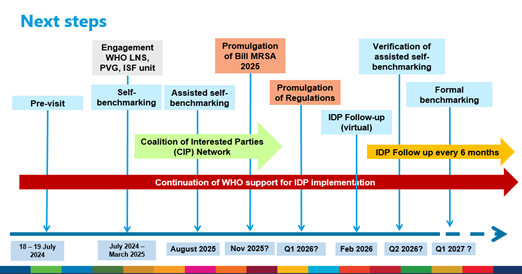
The exercise followed on the WHO introductory visit (pre-visit) to Botswana from 18 to 19 July 2024 to explain the WHO regulatory system strengthening (RSS) programme, and the overall benchmarking process. During the WHO assisted self-benchmarking process, prepared, organized and conducted as per the Quality Management System principles, each sub-indicator of the WHO global benchmarking tool (GBT) was scored, the strengths and any existing gaps were identified, after which BOMRA elaborated the institutional development plan (IDP) designed to address the identified gaps. The status of the regulatory system regarding its maturity level for all regulatory functions was updated. The regulatory functions covered included National Regulatory System (RS), Regulatory Inspection (RI), Registration and Marketing Authorization (MA), Laboratory Testing (LT), Licensing Establishment (LI), Vigilance (VL), Clinical Trial’s Oversight (CT) and Market Surveillance & Control (MC).
The BOMRA assisted self-benchmarking exercise builds on the progress made following the SADC self-benchmarking workshop in April 2019 and July 2021 (GBT+ Blood) and the self-benchmarking activities in February 2020, April 2021 and March 2022. The overall outcome of the assessment indicates that the operationalization of all the regulatory functions has made significant progress since BOMRA was established. The Licensing Establishment (LI) function was scored at WHO Maturity Level 3. The rest of the functions were scored at WHO Maturity Level 1 because of the pending Medicine and Related Substances Bill, 2025. The Bill is scheduled to be tabled in the Parliament in November 2025.
Among the strengths, the assessors noted a high level of technical competence across regulatory functions, a commitment to consistent regulatory oversight (quality, safety, regulatory compliance) by licensing publicly owned manufacturing facilities and that a fully institutionalized and functional Botswana Integrated Information Management System (BRIMS) will deliver significant efficiencies for regulatory activities. The political will to promulgate the Bill Medicine and Related Substances Act (MRSA), 2025 was noted underscoring the strong commitment to attain Maturity Level 3 and beyond. The assessors also noted that the Strategic Plan with defined objectives, KPIs and targets is already drafted.
However, in order to fast track the attainment of WHO ML3, the promulgation of Medicine and Related Substances Act, 2025 and Regulations should be realized before the end of 2025. Additionally, BOMRA should address the backlog in Market Authorization and Registration function that emerged as workload increased without corresponding increase in staffing levels, implement of the Botswana Integrated Information Management System (BRIMS) and update the Quality Management System. This will require mobilization of financial resources and having staffing levels commensurate with regulatory mandate and workload.
Speaking during the closing session, BoMRA CEO Dr. Seima Dijeng expressed gratitude to the WHO experts and the BoMRA team for their collaborative effort towards the journey to ML3. He emphasized the importance of understanding the status of the country’s regulatory system and addressing identified gaps and areas of improvement. He further commended the dedicated BoMRA team for their commitment and hard work, ensuring that the public has timely access to trusted medical products. The assessment signaled a positive outcome, indicating that the attainment of ML3 is on the horizon, a remarkable milestone for a regulatory authority that commenced operations only six years ago, in 2018. The progress reinforces BoMRA’s strategic priorities to align its regulatory framework with global health standards to safeguard public health. The journey to ML3 and beyond continues.